David Dodd: Thank you. And good afternoon, everyone. And again, thank you for participating in the 2022 first-quarter update call. First quarter marked a transformational period for GeoVax in that we achieved clinical stage development status within the two priority areas of COVID-19 vaccine development and cancer immunotherapy.
In addition, we added strong expertise and experience to ensure our ability to expand and accelerate our clinical development programs in the requisite activities through regulatory registration, manufacturing, and distribution. Our mission is to provide products to prevent or treat some of the world's most challenging infectious diseases and cancers, by leveraging technology and collaborations that allow us to successfully provide life-enhancing products in a safe scientific validated manner.
Our pursuit is to deliver safe, affordable products to improve lives worldwide, delivering increased value to our shareholders and providing motivating career development opportunities to members of our team. We firmly believe that GeoVax can provide differentiated, advanced vaccines and immunotherapies competing with and collaborating with other companies worldwide. We intend to successfully execute in this regard. We're pleased to have this opportunity to review our successful progress into Phase 2 clinical development, while securing critical resources in support of GeoVax's growth and development, while also advancing our IND-enabling programs.

The in-licensing of both GEO-CM04S1 and Gedeptin® represented watershed events of the transformation of GeoVax, as a milestone towards potential significant value expansion of the company. CM04S1, which we in-licensed exclusive global rights from City of Hope National Medical Center, is a next-generation COVID-19 vaccine, targeting both antibody and cellular immunity with the goal of providing more robust and durable protection than the current authorized vaccines. Gedeptin is a cancer immunotherapy which we in-licensed exclusive global rights from PNP Therapeutics to the University of Alabama, Birmingham, currently being evaluated among patients suffering from advanced head and neck cancers; it has already received orphan drug designation from the FDA.In addition, we're advancing encouraging internal programs on the path to the IND panel.

In January, we issued a 2021 Milestone Report addressing the goals we established and communicated early last year. That update outlined our successful 2021 performance in executing upon those goals, bringing us to the Phase 2 clinical status, enabling our progress related to our internal programs, while strengthening the organization with the expertise to accelerate our growth and development. At the 2021 World Vaccine and Immunotherapy Congress last December, we reported results validating our COVID-19 vaccine approach of a multi antigenic vaccine inducing strong antibody of cellular immune responses, potentially providing more robust and durable protection beyond the current authorized vaccines.
In fact, in a well validated lethal challenge transgenic mouse model, our CMO2 vaccine candidate, which is the first step towards a universal Coronavirus vaccine, provided complete protection following a single dose, even in the absence of measurable neutralizing antibodies. To our knowledge, these results are unprecedented. I'll note that both our Phase 2 COVID-19 vaccine, CM04S1, and our universal Coronavirus vaccine candidate, CMO2, are each multi-antigenic COVID-19 vaccines, designed to strongly induce both antibody and cellular immune responses.
At that same Scientific Congress, we also reported further encouraging results in support of our Marburg and Sudan vaccines. Last week, at the World Vaccine Congress, Dr. Newman further discussed the status of CM04S1 and our basis in support of our multi-antigen universal Coronavirus vaccine.

In January, we further strengthened our balance sheet and are currently well-capitalized for at least a year. We anticipate further strengthening of our balance sheet during 2022. Our primary focus this year is to accelerate the recruitment and enrollment of our three Phase 2 programs. This includes our multi-site clinical trial in support of Gedeptin and our two clinical trials in support of CM04S1.

Since acquiring the rights of Gedeptin, we've confirmed two additional clinical sites in the assignment of CATO SMS as our CRO partner responsible for leading the expansion acceleration of the Gedeptin clinical program. Our focus on accelerated and expanded patient enrollment is actively underway, with the goal to complete patient enrollment towards the end of 2022 or early 2023, followed by completion of patient evaluations, perhaps by the end of 2023. Should the results be supportive, a BLA filing will likely follow shortly thereafter.
In parallel with the ongoing clinical program, we are also engaged with a CDMO to prepare for commercial manufacture. We are confident that the Gedeptin Phase 2 program will be successfully managed by CATO SMS, and our clinical operations team with possible expansion of further additional clinical sites. We are highly excited about the outlook and promise of Gedeptin within advanced head and neck cancer, where it has received orphan drug designation and has previously provided encouraging potential for such patients.
In addition, there are promising opportunities relative to expanded use of Gedeptin in other indications as well as the GDEPT technology in conjunction with other therapies, and potential synergy with our MVA-VLP tumor associated antigen approach. We're looking forward to providing milestone updates throughout this year about the progress of our Gedeptin program.

Also, during the first quarter, we focused on the two Phase 2 clinical trials in support of CMO4S1. This vaccine utilizes synthetic Modified Vaccinia Ankara vaccine MVA technology similar to other vaccine programs under development at GeoVax. CM04S1 induces immunity to SARS CoV-2 by stimulating the immune system to produce antibodies against SARS-CoV-2 that can block the virus from entering healthy cells, while the immune system can also grow new disease fighting T-cells that can recognize and destroy infected cells.
The vaccine includes both SARS CoV-2 spike and nucleocapsid proteins. By inserting these proteins, the MVA delivery vehicle is able to drive the expression of both proteins within the body of the vaccine recipient, inducing immune responses. The role of the S protein is to elicit a neutralizing antibody response against the initial infection, while the N protein elicits or reduces a T-cell response to directly attack virus infected cells, reduce viral replication and reduce severity and clearance. Thus, the vaccine is designed to induce both neutralizing antibodies and T-cell responses specific for the S protein and the N protein. This vaccine design was implemented specifically to induce an expanded immune response to better combat and clear infections regardless of the circulating SARS-CoV-2 variance.
This vaccine is the first step in the worldwide goal to provide a vaccine that gets ahead of the variants versus having to chase the variants. If successful, this vaccine will reduce reliance on the repeated administration of booster doses of existing vaccines. We believe that a multi-punch approach has the potential for providing a more robust and durable immune response and protection than the current authorized vaccines. We also believe that very high risk populations such as immunocompromised individuals, will benefit from such a two-pronged approach.
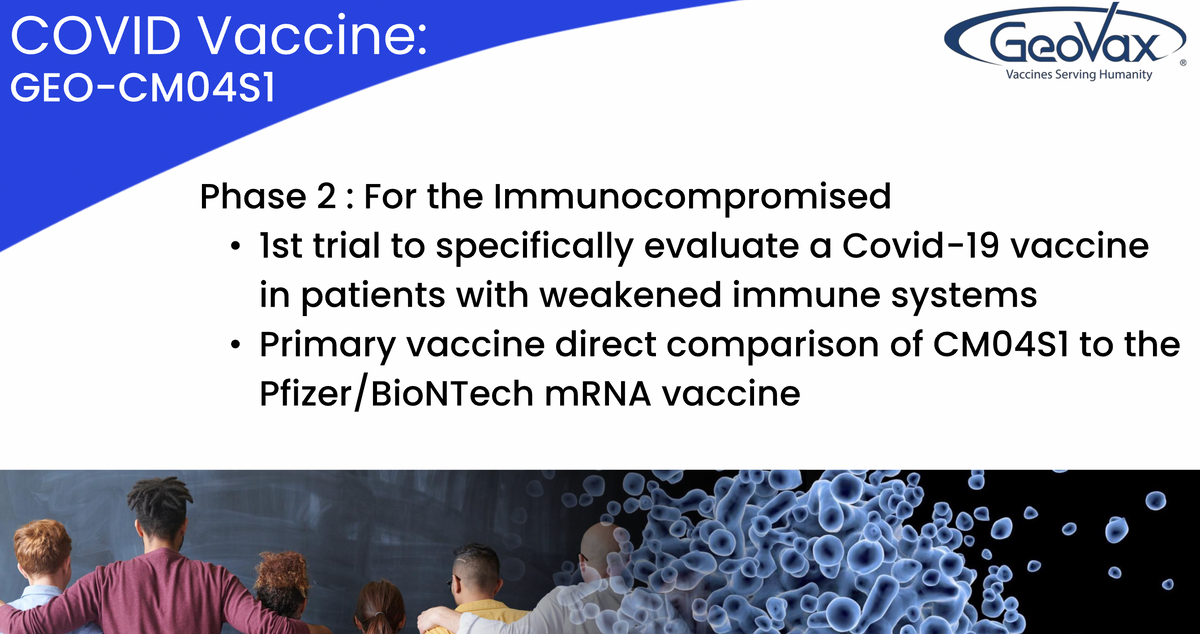
CMO4S1 is currently being evaluated in two Phase 2 clinical trials. One trial is the first comparative study of an investigational COVID-19 vaccine, as the primary vaccine versus the current FDA approved Pfizer vaccine and in individuals that have received or are undergoing specific blood cancer therapies associated with transplantation or CAR T therapy to suppress or severely reduce pre -existing immunity to COVID-19 vaccines. Multiple clinical evidence has demonstrated and validated that such patients failed to respond optimally to the current generation vaccine and we believe that CMO4S1 will prove to be the more potent vaccine because it is multi-antigenic and delivered using the MVA vector.
We believe this will differentiate CMO4S1 from the other vaccines by providing both a strong antibody response and a sustained T-cell response for these patients who are still at high risk of severe COVID-19 due to their immunocompromised status.

The other trial currently underway is evaluating CMO4S1 as a booster for healthy patients who have previously received either the Pfizer or Moderna MRNA vaccines. We believe that providing a heterologous booster rather than a third or fourth or fifth shot of the same vaccine may provide more robust and durable immune response and protection. Heterologous prime boost immunizations are well studied in other fields such as HIV and are being evaluated in multiple countries using different COVID vaccines. We are working with them now combined CATO SMS and Pharm-Olam to oversee the acceleration and management of these two exciting clinical programs working under the direction of our internal clinical operations team.
Finally, the ongoing GeoVax effort to develop a manufacturing process based on a continuously growing AVM cell line to increase production, consistency and capacity will mesh with the clinical development activities and full development schedule associated with CM04S1 and CMO2 vaccines.
David Dodd: My colleagues and I will now answer your questions and joining us as we've mentioned earlier, for the Q&A session, Mark Newman and John Sharkey, our Chief Scientific Officer and Head of Business Development, respectively. I'm therefore turning the call over to the operator for instructions on the Q&A session.

Operator: The first question will come from Jason McCarthy with Maxim Group. Please go ahead.
Joanne Lee: Hi, this is Joanne Lee on the call for Jason. Thanks for taking the questions. I wanted to congratulate you guys on the recent publication of the Phase 1 data of all 04S1 last month. As a reminder, could you just walk us through how the results from that trial have highlighted the vaccine's mechanism of action and its differentiation from the current vaccine?
David Dodd: Sure, I'll ask Mark Newman to step in on that.
Mark Newman: Sure. The…I mean I think that it's fairly straightforward as David said, that vaccine was designed to induce antibody responses and T-cell responses with the primary antibody target being the S protein and then T-cell responses would be generated to both the S and the N. And we measure the antibody responses by enzyme linked immunoassay ELISA, which is just total titers and neutralizing function. And both of those are…were readily detectable and at levels comparable to what you're seeing with the approved vaccines plus or minus the…a range. It's a fairly small study.
And then we're also seeing the T-cell responses, which we typically measure as cytokine production. They're what's called the type 1 response, which is the predominant effective antiviral response. Whereas the type two response would be considered more for allergies and things like that. So, it's the correct type of response. The nice thing is we saw good responses at all dose levels tested, that would be the dose escalation trial as most Phase 1 trials are safe at all doses and the lower dose was essentially as immunogenic as the middle and higher doses. We've got some range to work with. Does that help?
Joanne Lee: Yes. Great. That was helpful. So, for your two ongoing programs, 04S1 could you just give us any update on timelines in terms of the enrollment status? And lastly, as a follow-up, if you could briefly touch on, given the way new variants have been emerging and driving a constant ongoing string of boosters with the same vaccine, could you just touch on the importance of getting new vaccines to market that have differentiated mechanisms and targets? Thanks.
David Dodd: Okay. Mark, you may want to comment on it, but I think Kelly McKee, Dr. McKee, our Chief Medical Officer, may want to add some comments also on that. So, I'll leave it up to you two, to go…whoever wants to go first.
Mark Newman: Okay. Well, let me start. So, we…you've heard us talk for a year that we were not really chasing variants. The Omicron was just a perfect predictable next-generation variant, right? It… we're seeing immune pressure against the circulating virus and now the new variants are coming up and there's less and less protection from the neutralizing antibodies. And of course, the mutations are happening in the S protein primarily because that's where the antibodies are targeted. So, I think that we're in the right position. If you follow the field, you will have noticed that there was an open letter recently sent to the FDA Commissioner highlighting the importance of T-cells and imploring the FDA to ask vaccine manufacturers to start looking at T-cell responses. And I think that that made the Wall Street Journal and we're there already. So, I think we're in the right position. The world is starting to recognize the importance of a broader immune response. World Health stepped in, everyone is stepping up and saying, well, we'd like something that is variant proofed. The terms universal Coronavirus or cross variant, or pan-sarbecovirus virus are all being bandied around quite a bit and this has been a core part of our program since the start. And so, with the first two generations…with the first-generation with two candidates one in Phase 2 and one in animal testing both proving the highly immunogenic and effective in reducing types of responses. We're well along the way with this first step towards making a very improved and a more potent next-generation vaccine.
Mark Newman: Kelly, do you want to add something?
Kelly McKee: Yes. Hello everyone. Not so much on the scientific side, but I can help out a little bit with the status of the clinical trials. As you may be aware, that the…our study in the immunocompromised populations, the transplant trial, the study design is somewhat complicated, but I will say that we've enrolled patients in all of our different cell therapy cohorts, and we're sort of in the process of completing the safety lead in phase in those cohorts. And for one of the cohorts, we're just about ready to transition into the blinded portion of the trial to compare against the Pfizer vaccine.
Now, one of the challenges that we've faced with that trial is the evolving therapeutic landscape for immunocompromised patients, and so we've been…it's been necessary for us to modify the protocol progressively to ensure that we're compliant with the standard of care for vaccination of these patients. And between the regulatory timelines connected with protocol amendments and so forth, the enrollment has not been quite as fast as we would like, but it's still progressing pretty well. The healthy volunteer study, a similar sort of situation. It's a more straightforward trial, but again, in order to sort of keep…to comply with the current practice of boosting healthy individuals, we're modifying that protocol to enable more aggressive enrollment and we're very hopeful that that's going to pick up very quickly as well.
Joanne Lee: Got it. Thanks again for taking the questions.
Kelly McKee: Thank you.
Operator: The next question will come from Jeffrey Kraws with Crystal Research. Please go ahead.
Jeffrey Kraws: Thank you very much. David, it looks like this might be either for Sharkey or Newman possibly. But looking at Gedeptin, I realize that there's a large focus in the marketplace on the COVID vaccines, but Gedeptin seems to have incredible potential. When I was looking at some of the scientific research on this, you know, in conjunction with checkpoint inhibitors, it seems to have interesting enhanced performance of the checkpoint inhibitors. One, can someone expound a little bit about that?
And secondly, can you provide an update on the status of the trial, as well as some of the opportunities that I think, lay ahead of us? Those are the first couple of questions.
David Dodd: Okay. Thank you, Jeff. John, why don't you take the first part, and then Kelly can give an update on the progress on the clinical or Mark Newman, would you...I don't know if John is still on?
Mark Newman: Well, yes, I can start. I think John's muted probably. So yes, we're familiar, obviously familiar with the checkpoint inhibitor story, and I think that any product that can make the checkpoint inhibitors work better against solid tumors, particularly at adenocarcinomas has great potential. So that's not part of the initial focus, but it is something that is being evaluated by a collaborator at Emory University. And there is a lot of interest in the field with combining all of these different products with checkpoint inhibitors compared with the standard of care essentially. I mean, that's when we look at the cancer market, and the approach, everything is in addition to standard of care.
So I don't think that the vaccines would be anywhere near as exciting, nor would these other types of products be anywhere near as exciting if you couldn't plug them in with the checkpoint inhibitor. So ideally the goal here is that Gedeptin will kill tumors. And we presented this before. And as the tumors are dying, you release neoantigens and the neoantigens are the basis of a vaccine. So it's an autologous vaccine concept. We're not the only ones that have looked at this and there's a lot of literature out there on that, but it's not the lead on the program right now, but it is something that's being investigated.
David Dodd: Let me just say, John, are you there or would you like to add something? If not, I'll ask Kelly to update on the clinical program. Kelly.
Kelly McKee: Sure. No, I guess John is having trouble with his audio. The clinical program as David indicated in his opening remarks, where we're...as we've transitioned the IND over to GeoVax, we've expanded the trial from the original single site at Stanford and added two more study sites, one at Emory and one at Thomas Jefferson University in anticipation of being able to accelerate the enrollment. At the present time, we're about halfway through enrolling our total target number.
And just as soon as we get all of the regulatory pieces back in place, which shouldn't be too much longer from now, we should begin. We've already got a couple of patients actually identified as potential candidates, but we should begin re-enrolling and finishing up that trial very quickly.
Jeffrey Kraws: Thank you very much. And lastly, recently there was a letter signed by about 100 scientists, one of which was, I believe, one of your consultants. Can you talk about the significance of that, as well as the fact that so many people seem to be, I won't say jumping on the bandwagon, but providing support which is congruent with your thoughts on many aspects in the science, David?
David Dodd: Sure. Now, Mark, you want to mention about that, because that's one that Don Diamond who led the development of CM04S1 out of City of Hope. He was also a signatory on that correspondence.
Mark Newman: Yes. Sure. And that's the letter I referred to just recently in an earlier question. So this is a number of immunologists or medical professionals working in the vaccine field that recognized that focusing solely on antibodies is an issue. And the issue is, is that that's not the total immune system. The immune system involves a complex, coordinated set of effector functions, and antibodies are only a single one. I thought what was really interesting about the letters; they actually cite the fact that there were some deficits impacting how the FDA reviews, for example, pediatric use of vaccines because they're only focusing on antibodies. And, in fact, maybe had they...had rolled in a T-cell function...a measure of T-cells they would've seen that the pediatric efficacy looks better.
The world...the FDA is...it's hard to predict, but there is a move in the field to start looking at surrogate markers more and more, and that means you're not looking just at efficacy. If you remember the initial trials, there are 30,000 plus patients in each of these trials for the products that were approved or got emergency use authorization. And they're looking for either they're infected and end up with symptoms or they're not as classic efficacy. It's now recognized that placebo-controlled blinded efficacy studies like that just aren't the way to go as we move forward attacking the new variants and adding new vaccines. And so, there's always...there's a series of meetings that are ongoing every couple of months, most of them run through World Health Organization, the blueprint series, where these types of topics are discussed. So I think that this letter just fits in with a lot of other things that we're seeing, and that is a recognition that we've now...we've got some place, we've got great progress, but now we have to move back or move forward with the next step. And that's going to include a more broadly base characterization of immunity and testing of vaccines that have slightly different functions.
I think, like David said, we had a meeting, just...a conference just last week and the closing session was can we make a universal COVID vaccine? And the CEPI representative pointed out, well, something that's variant-proof would be great, something that stops the next spill over from bats would be tremendous. And so, that's where we're moving. And that's going to involve T-cells. And that's where I think you're starting to see momentum.
Mark Newman: Well, I hope… Go ahead, David.
David Dodd: I was just going to say, and that's what we're focused on as a very strong differentiated point is about the focus also on the T-cells. And I think that's where we'll be able to carve out some very important areas of opportunity for our vaccine. Jeff, you had asked and was going to ask if John Sharkey, who I think has been able to rejoin us now. If John wanted to comment on some of the animal work done at Emory, with Gedeptin in conjunction with checkpoint inhibitors.
John Sharkey: Sure. Sorry, for some reason I was disconnected, but yes your question was about some of the results we're seeing, and what's been observed is that we take a humanized tumor that's resistant to checkpoint inhibition and transplant that into a rat model in both the left and right hind quarter. We then treat the left hind quarter tumor with Gedeptin followed with fludarabine phosphate, so we essentially are just pulling that tumor apart. We then come back with a checkpoint inhibitor. Not only does the left hand tumor respond to the checkpoint, but the right hand tumor which never received the Gedeptin also now responds.
And this is a hypothesis that is releasing the new adjuvants and other things from the left hand tumors as it's broad and that's now sensitizing or messaging the right hand tumor. And the way to tumor spots, it has been known for a long time. You recall back many years ago, one found that if you had metastatic disease in a model on top there's a primary tumor…would grow. So we know these tumors talked to others. Preclinical data is highly interesting, because it does lead that would carry over into clinical trials. You would now have a treatment that could address solid tumors and their meds and would not need to be accessible only for some of the tumors…very interesting.
David Dodd: Thank you, John.
Jeffrey Kraws: That's very impressive. Thank you. And I guess my last comment on COVID. It's more of a comment than even a question, but if there's anything that you can expound upon, it would be appreciated. Having two members of a family that I know who both are going through chemo and had really nasty...one is still going through a really nasty bout with COVID obviously because of their compromised immune system. I would think that one of the targets...just not looking at the COVID vaccine...everybody talks about a different approach, but I would think these people who have compromised immune systems, that the multiple antigen approach would really help those individuals. And while it's not something that people focus on in the marketplace, obviously, there are a tremendous number of people suffering from cancer and going through...going through chemotherapy who are subsequently getting COVID and unfortunately a significant number of them are dying.
David Dodd: Kelly, would you like to comment on that?
Kelly McKee: Well, that's...you're exactly right. And I think the literature is replete with data to support the fact that immunocompromised, particularly severely immunocompromised individuals are much more susceptible to developing severe disease and dying from COVID infections and that's precisely why we feel that the CM04S1 vaccine offers, at the current time, unique option for protecting these people from COVID infection. Now, of course, it remains to be seen whether our hypothesis holds true, but at least in theory, if we can stimulate T-cells to a degree that will provide some sort of functional protection against the infection...against disease once these patients are infected, we think we've got something very important to offer.
David Dodd: Thank you very much
Mark Newman: Can I add something?
David Dodd: Thanks for the updates.
Mark Newman: I've got a paper on my desk titled “CD8 cells contribute to the survival of COVID-19 patients with hematologic cancers.” And there's a sentence in here that points out cancer patients have increased risk of severe COVID, estimated case fatality rate of 25%. That's ten times the general population. This is a big met analysis. So it's clearly a focus that needs to be addressed. And one of the things that people keep forgetting is that this MVA vector we're working with was developed as a super safe smallpox vaccine that is highly effective in populations like immunocompromised. So it's really a T-cell and an antibody driving type of vector with probably more T-cell power than the other vectors. And so, we really do think it's a good fit for these immunocompromised patients, and not just cancer patients, but people that are older, all sorts of immune compromised situations.
Jeffrey Kraws: Well, thank you very much for the updates.
David Dodd: Thank you.
Operator: The next question will come from Michael Okunewitch with Maxim Group. Please go ahead.
Michael Okunewitch: Hey, guys. Thank you for taking my question. So I'd like to ask about some of the differences in the approach between CM04S1 and CM02 that makes one a variant proof and the other potentially universal Coronavirus vaccine. Could you talk a bit about the targeting strategy for those two different vaccines?
David Dodd: Sure. Mark Newman, do you want to discuss that?
Mark Newman: Sure, I can address that. So...the basic similarity is multi-antigen. So we're mixing the antibody target, the S, with another structural protein. Now, these were developed initially independently, so one was City of Hope. That's the 04S1, so that's S plus N, and then our internal program is S plus M, which is membrane and envelope, and that...those are the components that are required for...the basic components required to have the Envivo virus like particle assembly that...kind of the basics of the GeoVax platform.
So the in-licensing, I mean, it was in-license because it was very similar. It was in clinical trials and we viewed it as a first step. And that's Phase 2, whereas the other program is in research. And just my own personal preference, we're not going to mess with the Phase 2 product right now. It is what it is and we want to see where we can take that. And so, we'll do more of the basic research with the CM02. That's just in animal testing right now. So that's the one where we can add additional proteins. We'll see how many different products...viral gene products that we can put into this vector to really broaden the response even more.
Again, if you follow the field, a lot of reports are coming out now about people that have been exposed to other Coronaviruses. They cause colds, just they're not deadly. And these have induced T-cell responses that help protect to some degree against COVID. And so, there is a continuing development of more and more data now showing that you're getting these kind of cross-reactive T-cell responses that will add to protection. So that's the kind of work being done with the CM02, but again, that's just in the mouse model right now.
Michael Okunewitch: Thank you. And then I'd also like to follow up, if you could discuss some of the difficulties of conducting a COVID trial at the moment, just given where we are in terms of cases and how the fluctuation seemed to be largely driven by emergence of new variants?
David Dodd: Sure, I'll make a comment and then I don't know Kelly, maybe you'll pick up from there. I would just say that my observation is that conducting clinical trials now versus let's say, a year or two...a year and a half ago is challenged by the fact that we do have other vaccines out. We've got Therapeutics out, the standard of care is evolving. And yes, you also are seeing continued evolution of this virus and emergence of new variants of concern. Kelly, do you want to pick it up from there?
Kelly McKee: Yes, sure. I think this speaks to some of the comments that Mark Newman made earlier around the need for us to sort of evolve thinking around what constitutes a surrogate marker for vaccine efficacy. And back in the days where we could evaluate protection by a clinical response. That is not only increasingly more difficult, it's not even possible. And so now, we're faced with the dilemma of what sorts of markers do we utilize in order to demonstrate that the next-generation vaccine be used as a primary vaccination or as a booster...what kind of marker will be required for us to demonstrate that we've got sort of a better mousetrap.
I think that the study designs themselves are probably pretty apparent. I mean, there will be non-inferiority types of comparisons to be made against existing authorized or licensed vaccines. But what measures we will use to compare against these vaccines...these current vaccines sort of remain to be resolved. And I think they are going to...I think our expectation is they're going to be as Mark indicated earlier, they're going to be an acceleration of talk and discussion with regulators to resolve this issue.
You may be aware that the NHRA, the regulatory agency in the United Kingdom recently approved Valneva's COVID-19 vaccine on the basis of the serologic marker, a non-inferiority against the existing AstraZeneca vaccine. And I think that's probably just the first in a number of regulators thinking the first in line for thinking by regulators about how to approach this problem.
David Dodd: And I would add Michael, if you think about what GeoVax has focused on as a strategy. We're looking to be able to differentiate, and we believe that CM04S1 may well demonstrate this…Is these populations as we've been talking about immunocompromised that represents depending on how you define it. But if you take people being treated for various cancers or dealing with that, people who've had genetics...who have genetic conditions of multiple sclerosis, who have weakened immune systems, people who have organ transplantation or immunosuppressive drugs, the very elderly, people with strong comorbidities. We begin talking anywhere from 10 million to 15 million of patients that are out there that clearly are not being adequately served by the existing vaccines.
And then if you look at the opportunity which are niche markets but very valuable and important ones from the standpoint of the patients and the size of those opportunities, but the same time in a greater sense, is this need to have a booster that give greater durability, because we continue to see an unacceptable waning of the level of protection of the immune response that's coming from the existing, just giving another shot on top of the same shots. So we think there are a lot of areas that need to evolve. Clearly how...the discussions about how to evaluate and measure those as Kelly was just talking about is absolutely critical and that's going to evolve significantly also.
Michael Okunewitch: Alright, thank you very much. I really appreciate your insights. Thank you.
Operator: This concludes our question and answer session. I would like to turn the conference back over to Mr. David Dodd for any closing remarks. Please go ahead.
CONCLUSION
David Dodd: Thank you, everyone. Not only for participating in this conference call, but also for providing some very good, insightful questions, and giving us an opportunity to discuss why we're doing what we're doing and what we are anticipating what we might well see coming out of the clinical end. But our focus is clearly as we've said, it's now in execution and reporting updates and progress for Gedeptin, CM04S1, CM02, and our other development programs, as well as updates on the expansion of our capabilities and resources.
As I noted at the beginning of today's presentation, our pursuit is to deliver safe, affordable products to improve lives worldwide. The products like, vaccines and immuno-therapies that can be not only delivered, but administered and utilized in wherever they need to be and increasing value to our shareholders and again, providing motivating career development opportunities for the members of our team.
We also want to acknowledge and thank our board of directors, our staff, and the many other parties that continue to support, assist, and advise us towards achieving success. For us, it's a great pleasure to be able to give you these updates and hopefully excite you about our company and for being a part of this team. So have a safe, enjoyable day. This concludes our conference call. Thank you.
GeoVax Labs, Inc. is a clinical-stage biotechnology company developing human vaccines and immunotherapies against infectious diseases and cancer using novel proprietary platforms. GeoVax’s product pipeline includes two ongoing Phase 2 clinical trials of GEO-CM04S1 for COVID-19 as a universal booster vaccine to mRNA vaccines authorized by the U.S. Food and Drug Administration (FDA) and as a primary vaccine for use in immunocompromised patients. In addition to GEO-CM04S1 for COVID-19, GeoVax is developing GEO-CM02 as a pan-coronavirus vaccine. The Company is also conducting a Phase 1/2 clinical trial of Gedeptin® for treatment of head and neck cancer. Gedeptin® has been granted orphan drug status by the FDA. Additional research and development programs include preventive vaccines against Zika Virus, hemorrhagic fever viruses (Ebola, Sudan, Marburg, and Lassa) and malaria, as well as immunotherapies for multiple solid tumors. The Company’s portfolio of wholly owned, co-owned, and in-licensed intellectual property stands at over 70 granted or pending patent applications spread over 20 patent families.
This release contains forward-looking statements regarding GeoVax’s business plans. The words “believe,” “look forward to,” “may,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,” “could,” “target,” “potential,” “is likely,” “will,” “expect” and similar expressions, as they relate to us, are intended to identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. Actual results may differ materially from those included in these statements due to a variety of factors, including whether: GeoVax is able to obtain acceptable results from ongoing or future clinical trials of its investigational products, GeoVax’s immuno-oncology products and preventative vaccines can provoke the desired responses, and those products or vaccines can be used effectively, GeoVax’s viral vector technology adequately amplifies immune responses to cancer antigens, GeoVax can develop and manufacture its immuno-oncology products and preventative vaccines with the desired characteristics in a timely manner, GeoVax’s immuno-oncology products and preventative vaccines will be safe for human use, GeoVax’s vaccines will effectively prevent targeted infections in humans, GeoVax’s immuno-oncology products and preventative vaccines will receive regulatory approvals necessary to be licensed and marketed, GeoVax raises required capital to complete development, there is development of competitive products that may be more effective or easier to use than GeoVax’s products, GeoVax will be able to enter into favorable manufacturing and distribution agreements, and other factors, over which GeoVax has no control.









