Good afternoon and thank you for participating in the GeoVax corporate update call. The third quarter represented continued progress for GeoVax as we advanced the phase 2 clinical programs in support of Gedeptin®, our cancer therapy for patients with advanced head and neck cancers, and GEO-CM04S1, our next generation COVID-19 vaccine. In addition, we continue to progress our preclinical development stage programs. We also further strengthened our balance sheet during a very difficult investment environment, especially for the biotech industry.
As a result of our successful financings this year, we are well capitalized to complete our current phase 2 clinical programs, including expansion of the Gedeptin, multi-site trial and expanding the CM04S1 COVID-19 vaccine trial among immunocompromised patients to additional sites. We are focused on accelerating our programs with anticipated, initial data readouts over the next six to nine months.
Our mission is to provide immunotherapies and vaccines that improve lives worldwide, preventing or treating some of the world's most challenging cancers and infectious diseases. Also, we recently initiated business development discussions towards partnering and collaborations to ensure worldwide access to our products and up value to our stakeholders.

Gedeptin and CM04S1 provide significant value expansion opportunities for the company, our shareholders, and our stakeholders. As a reminder, Gedeptin is a cancer therapy currently in an expanded, multi-site evaluation among patients suffering from advanced head and neck cancers. The product has received orphan drug designation from the FDA, as well as the initial funding and support of the current clinical trial coming from the FDA orphan drugs clinical trials program.
Our focus is on accelerated and expanded patient enrollment, now that additional Gedeptin sites are on board. Our goal is to complete patient enrollment in 2023, followed by completion of patient evaluations before the end of 2024. In the interim, we expect to engage with the FDA regarding the results with the focus on clarifying the opportunity for an expedited biologics license application filing. We're excited about the outlook and promise of Gedeptin within advanced head and neck cancers.
In addition, there are promising opportunities relative to expanded use of Gedeptin in other indications, as well as application of the underlying technology in conjunction with other therapies and potential synergy with our internally developed MUC1 tumor-associated antigen approach. We also anticipate expanded discussions related to partnering and collaborations related to Gedeptin.

CM04S1 is our leading, next-generation COVID-19 vaccine, currently in phase 2 clinical development, targeting both the antibody and cellular arms of the immune system, with the goal of providing more robust and durable protection than the currently authorized vaccines. This vaccine holds promise over the current authorized vaccines from several critical areas of differentiation and value to various patient populations. Our focus is on accelerating the clinical development of each of these products, including the potential for expedited regulatory review. Meanwhile, we continue to advance other internal development programs in the path to IND filing.

We remain focused on accelerating efforts towards our milestones over the next 12 to 18 months in support of both the Gedeptin and the CM04S1 COVID-19 vaccine programs. We expect to report not only on the expansion of the Gedeptin study to additional sites, but also initial clinical data which we anticipate reporting during the first half of 2023. We also anticipate reporting initial clinical results in support of CM04S1 during the first half of 2023.
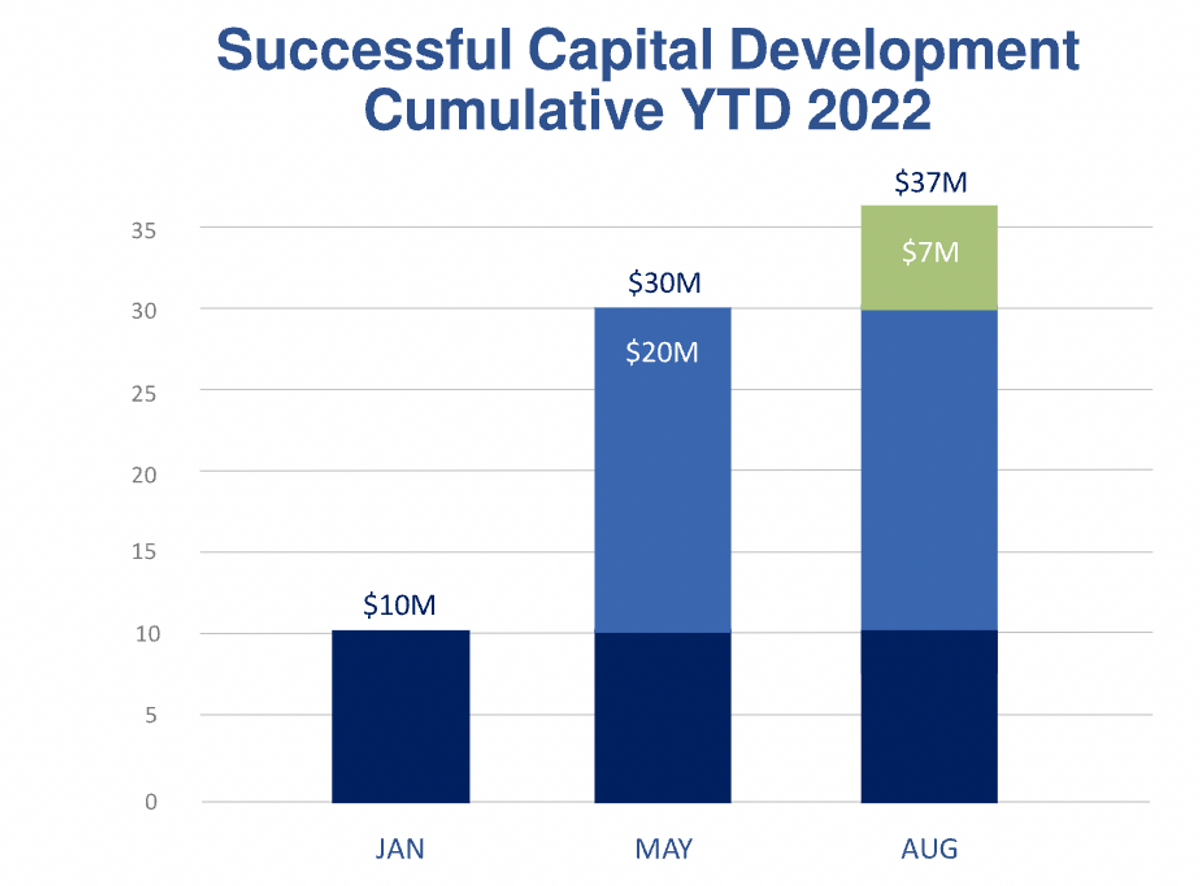
In addition, we will continue to provide updates related to the progress of our programs that are advancing the clinical status. Since January, we have added $37 million to our balance sheet, despite this year representing what many consider the most challenging year in over a decade for capital development within the biotech industry. Our successful financing supports efforts towards the completion of the current clinical programs.
We continue to receive strong interest related to investment capital, which we'll evaluate, but we're focused on execution towards our 2022 and 2023 goals of building shareholder value.
We're committed to the clinical development of CM04S1 against COVID-19, specifically as an invaluable medical tool to address the continually emerging variance of concern while also providing critically important value to those with compromised immune systems.
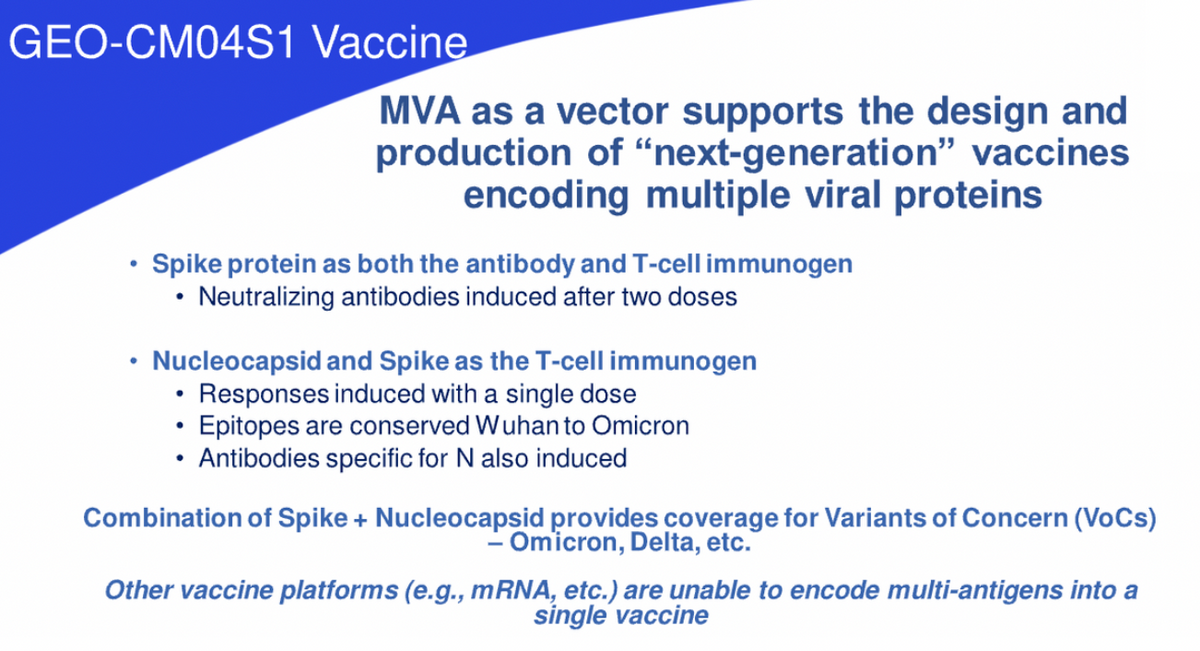
CM04S1 induces immunity to SARS-CoV-2 by stimulating the immune system to produce antibodies that can block the virus from entering healthy cells, while also inducing the growth of new disease-fighting T cells that can recognize and destroy infected cells. This is an important distinction between CM04S1 and the first generation COVID-19 vaccines. Our vaccine includes both SARS-CoV-2 spike and nucleocapsid proteins as immunogens differing from the current authorized vaccines, which only include the spike protein.
By inserting both of these viral genes, the vaccine is able to drive the expression of both proteins within the body of the vaccine recipient. Again, this is an important distinction as CM04S1 is specifically designed to induce an expanded immune response, offering significant potential for improved levels of efficacy and differentiation within the field. This vaccine design was implemented specifically to induce an expanded immune response to better combat and clear infections regardless of the circulating SARS-CoV-2 variants. The vaccine is the first step in the worldwide goal to provide a vaccine that actually gets ahead of the variants versus having to chase the variants.

David Dodd: Our approach of using the spike and nucleocapsid viral proteins was recently validated by multiple independent academic research groups and animal models using MVA and other vaccine platforms. Assuming continued success, this vaccine will likely reduce the requirement to continually adjust the vaccine and the reliance on the repeated administration of booster doses. This is what we mean in stating getting ahead of the variants versus chasing the variants.
In July, analysis of data from the phase 1 study of CM04S1 published in the peer-reviewed journal iScience showed that the vaccine, which is based on the same Wuhan strain as the first generation approved vaccines, induced potent and equivalent T-cell cross reactivity against Delta and Omicron variants. These findings suggest that T-cell immunity, simulated by CM04S1, may constitute a critical line of defense to provide long-term protection against SARS-CoV-2 variants.
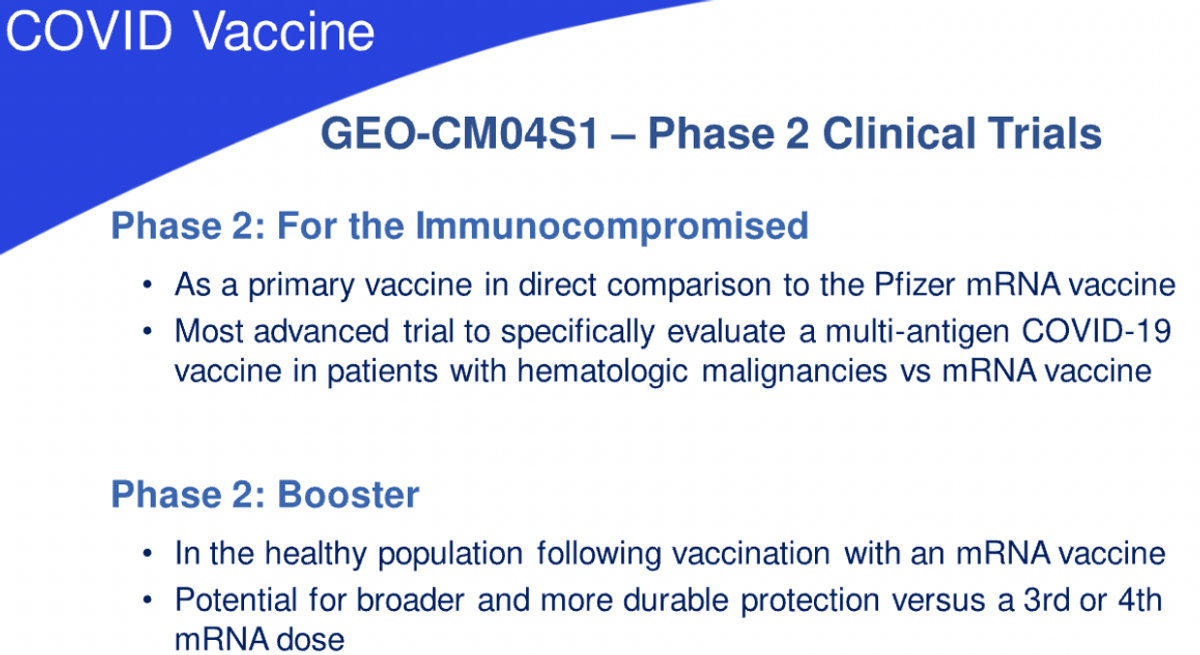
We also believe that the multi-punch approach has the potential of providing a more robust and durable immune response and protection in various high risk populations, specifically immunocompromised individuals, who will benefit from such a multi-pronged approach, resulting in broader and more durable protection. The vaccine is currently being evaluated in two, phase 2 clinical trials. One is a comparative study of our vaccine as the primary vaccine versus the current FDA approved Pfizer vaccine in individuals that have received or are undergoing specific blood cancer therapies associated with transplantation or CAR T therapy, which severely reduced preexisting immunity to COVID-19 vaccines.
Clinical evidence indicates that such patients fail to respond optimally to the current generation vaccines and we believe that CM04S1 will prove to be more potent because it is multi-antigenic and delivered using the highly potent MVA viral vector. We believe this will differentiate CM04S1 from the other vaccines by inducing strong and sustained antibody T-cell responses in patients who are at severe risk of COVID disease, due to their immunocompromised status.
The other phase 2 trial underway is evaluating CM04S1 as a booster for healthy patients who have previously received either the Pfizer or Moderna vaccines. We believe that providing a heterologous booster rather than continued multiple shots of the same vaccine will induce more robust and durable immune responses and protection. Heterologous prime booster immunizations are well-studied in other fields, such as HIV, and are being evaluated in multiple countries using different COVID-19 vaccines. Our goal is to provide a vaccine that ends the requirement of multiple boosters throughout a year.
Finally, I want to reinforce the concept that our internal effort to develop an advanced MVA manufacturing process based on continuously growing avian cell lines to increase production, consistency, capacity, and flexibility will mesh with the clinical development activities. In total, this represents the full development scope associated with the GeoVax coronavirus vaccines.
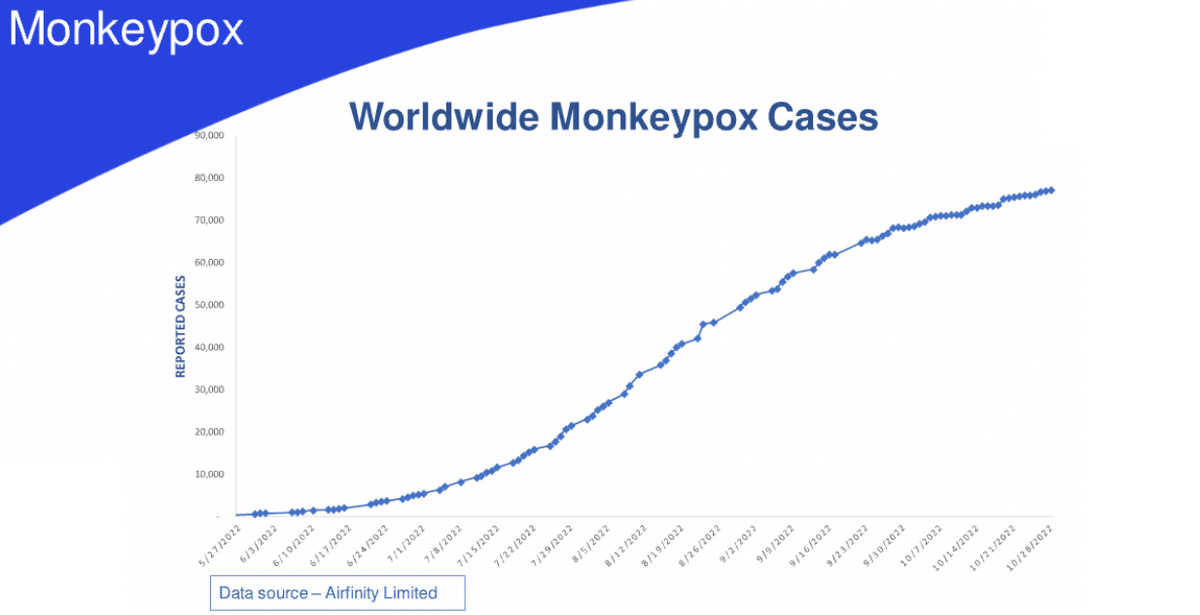
Monkeypox cases continue to increase worldwide with nations enacting procedures and policies in support of minimizing the health risk to their populations. There's also concern that more virulent and infectious forms of monkeypox might evolve, resulting in an increased spread of disease and deaths. The vaccine vector, modified vaccinia Ankara, or MVA, which is utilized in numerous GeoVax vaccines, including our CM04S1 COVID-19 vaccine, is the primary vaccine used to prevent the global monkeypox threat.
As you may know, MVA is currently stockpiled and utilized to protect against both monkeypox and smallpox.

In August, our colleagues at City of Hope published results demonstrating that both CM04S1 and the synthetic MVA used in the construct of CM04S1, induced robust antibody and cellular immune responses against monkeypox that were durable for over six months post-vaccination. The authors also concluded that CM04S1 and synthetic MVA represent unique vaccine candidates to control the expanding global monkeypox outbreak.
GeoVax is evaluating development regulatory pathways towards this opportunity as the need for expanding the MVA vaccine availability beyond the current limited, single-source provider is a global and increasing concern. And in fact, just today, we've announced our securing the rights of the NIH MVA for use against monkeypox and smallpox.
Now I'd like to turn the presentation over to Mark Reynolds, GeoVax Chief Financial Officer, to review our recent results and financial stats.

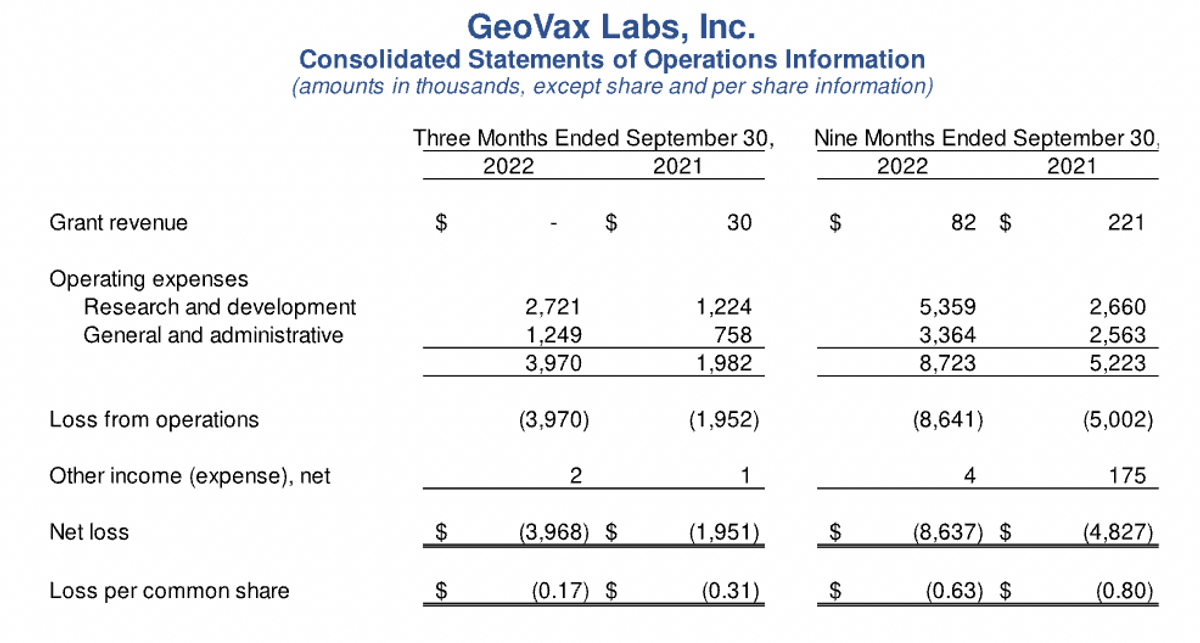
Thank you, David. So starting with our income statement, the first thing to note is the minimal amount of grant revenues reported during 2022 as compared to 2021, which is reflective of the expected wind down of our grant from the U.S. Army and the NIH for our Lassa fever programs and our COVID-19, preclinical program as we've now focused moving our attention to the clinical programs for both COVID-19 and Gedeptin. We intend to seek additional non-dilutive funding for our development programs in the future, and I'll make that point.
R&D expenses, or research and development expenses, were $2.7 million for the third quarter of 2022, and $5.4 million for the nine-month period, reflecting a substantial increase versus 2021 amounts, of $1.2 million and $2.7 million, respectively. These increases were planned and expected as they are associated with new clinical trial activity for our CM04S1 and Gedeptin programs, including manufacturing costs for clinical trial materials. The increase is also reflective of higher personnel and consulting costs as we staffed up earlier in the year.
General administrative expenses were $1.2 million for the third quarter of ‘22 and $3.4 million for the nine-month period, as compared to $758,000 and $2.6 million in 2021, with the increases, again, associated with higher personnel, consulting, and higher patent costs.
So overall net loss for the third quarter of 2022 was $4 million, or $0.17 per share versus $2 million in 2021 or $0.31 per share. For the nine-month period, our net loss was $8.6 million in 2022 or $0.63 per share versus $4.8 million in 2021 or $0.80 per share. Again, the increases during 2022 were primarily associated with the ramp up of our organizational infrastructure and other costs associated with the CM04S1 and Gedeptin clinical trials.
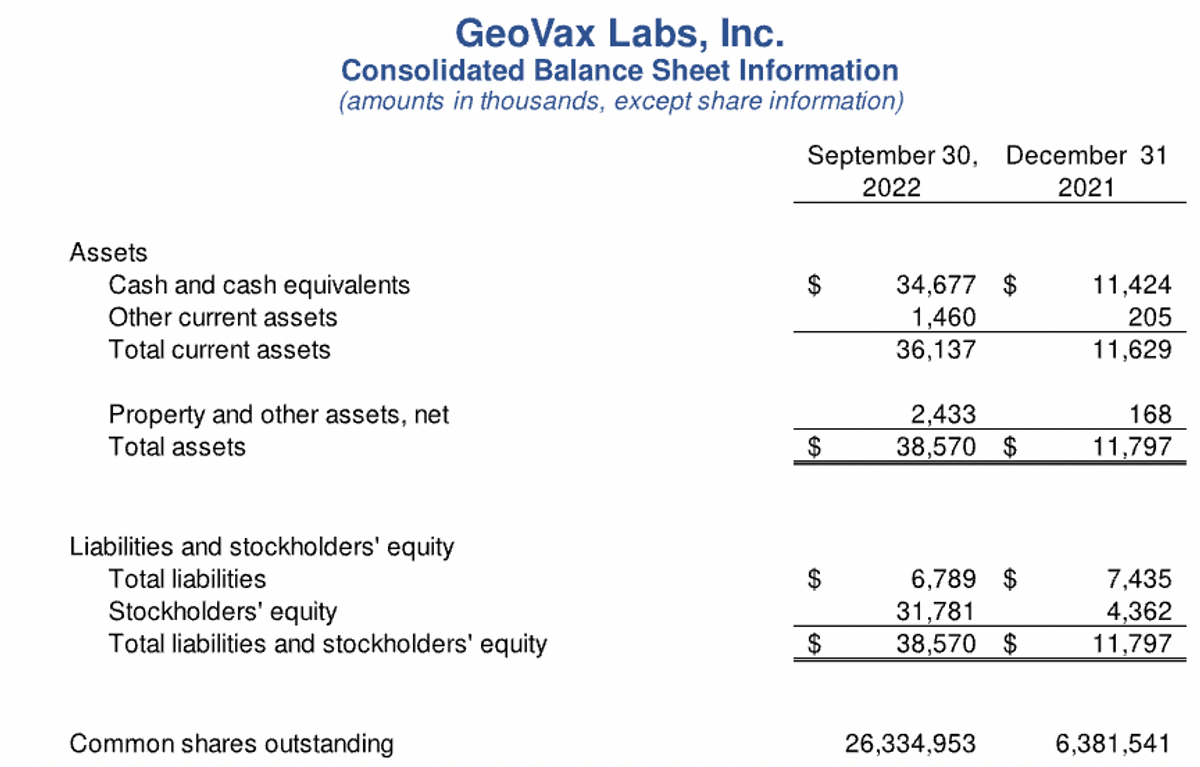
Turning now to the balance sheet, our cash balances at September 30 were approximately $35 million, as compared to $31 million at the end of the last quarter, and $1.4 million at the end of 2021. The change in cash balances for 2022 is reflective of $12 million used in operating activities, offset by proceeds from stock offerings in January and May, with combined net proceeds to us of nearly $28 million, and an additional $7.6 million proceeds from the exercise of warrants during the third quarter.
Our outstanding common shares now stand at 26.3 million. So in summary, we are well positioned to accelerate and advance our clinical programs with a cash runway sufficient to fund our operations and our priority programs through the end of next year. Funding our three, ongoing, phase 2 clinical programs and preparing for the next stages of development are the most significant use of our cash and our top financial priority.

David Dodd: Joining us for the Q&A session are, as mentioned earlier, Dr. Mark Newman, Dr. Kelly McKee, Dr. John Sharkey, our Chief Scientific Officer, Chief Medical Officer, and Vice President of Business Development, respectively.
Question 1- Jeffrey Kraws, Crystal Research Associates: Nice job on moving so many programs ahead and on schedule of what you had told us you were going to almost a year ago. I have a few questions. The first is on the COVID-19 immunocompromised trial. You had mentioned in your announcement that you were going to be expanding the number of centers. Can you please elaborate on how many centers you're planning to elaborate to and what we can expect out of that?
David Dodd: I'll ask Kelly McKee to address that. Dr. McKee?
Kelly McKee: Yeah, our current plan is to expand up to somewhere between 20 and 30 centers. It's a work in progress. We're making slow but steady progress with enlisting interested sites, but our ultimate goal is probably 20 to 30 sites.
Jeffrey Kraws: With your expertise in MVA right now and certainly the positive responses you're getting, what is the status rate now with regard to monkeypox?
David Dodd: I'll ask Dr. John Sharkey to address that. He is our point person on that. John?
John Sharkey: There are two aspects of the monkeypox, and I'll touch first upon the addition of a monkeypox indication to our MVA platform vaccine, such as COVID-19 and Ebola and the others. And we've been in discussions with our regulatory experts, mostly outside. We've come to the conclusion that there is a clear regulatory pathway to getting both the monkeypox and the smallpox indication and on to what we refer to as a primary MVA vaccine, such as our COVID-19 or an Ebola or something else.
As we work through this and, to put it simply, the primary vaccine indication, for example, COVID-19, will dictate the development timeline. But we believe, in parallel, we can collect the additional information required so that, at the time of registration, or quickly thereafter, we could add that indication. In regards to what we announced today with the rights to the NIH MVA for monkeypox and smallpox, we're looking to develop an MVA vaccine. We know MVA is effective here.
We believe that with the amount of information known about MVA, the safety profile of MVA, remembering that it was developed, used in immunocompromised populations, that there should be an abbreviated pathway, an expedited pathway to getting this through. With the MVA in hand, we started scaling up our master C batches, which would allow us to move into CGMP production and working with select regulatory agencies, we're going to map out, as I said, an expedited pathway to getting this to market. That's our intention.
Jeffrey Kraws: Last question, your head and neck centers, obviously, are active. Can you provide some of your thoughts as to what we should expect to see, as far as progress from those centers and will we expect to see you at ASH? Thank you.
David Dodd: Dr. McKee would you like to answer that -- respond, please?
Kelly McKee: So our ongoing Gedeptin trial now -- involves three, academic medical centers. The first one, Stanford, is the center that enrolled the initial five patients, and we've added two more. Both of them are now under contract, up and running, and actively seeking patients to screen and enroll. And we anticipate getting this study fully enrolled by the end of this year or at, the latest, early next year.
And as for ASH, I will be attending ASH, actually, so.
Question 2- Leah Lough, H.C. Wainwright: I was curious if you can provide us with some color on -- with what GeoVax thinks about recent results from competitors, specifically how it will relate to these immunocompromised individuals?
David Dodd: I would ask maybe Mark Newman -- Dr. Newman, you'd like to start off and maybe Kelly would like to then add on? And if I feel something else needs to be added- Mark?
Mark Newman: So when you say news from competitors, what specifically are you talking about?
Leah Lough: Well, I was just referring like, overall, say like the grand scheme. Right? There was just some news that came out from Novavax with the vaccine. Right? And just, overall, the question of, how should we be approaching a variant specific vaccine? And, I mean, all of this has been done in, not immunocompromised individuals. I'm just wondering, what does this mean for immunocompromised individuals? At least how does GeoVax see the landscape for that specific patient population?
Mark Newman: So, remember, the MVA was developed as a smallpox vaccine specifically for use in patients with some level of immunocompromised immune systems. So the safety and everything is unique, but it's also the potency. This is a product that is very effective at driving antiviral responses, particularly T-cell responses. And so I think there's somewhat of a unique niche in the way that the vector is used. You don't get this with, like an mRNA product, per se. There's none of the additional kind of antiviral effects to it.
Beyond that, if -- you probably recall, and we've been focusing on the concept of breadth of response. And so, the virus, coronavirus, does a lot of proteins and it's great to target just the S protein, but the immune system gets a chance to see everything. And so, we're more closely trying to mimic a normal response, something that you would see if you recover on your own. So it's the induction of an antibody response, not only to S, but also to N, the T-cell responses, as well.
And then, the independent research that's starting to get published kind of routinely now, both in animal models and in clinical trials, showing that immune responses that are more broadly targeted, S plus N, can actually protect animals from infectious challenge. This is, like I said, coming up from independent studies, which it -- but it’s really nice. It's validating what we've got into a phase 2 trial.
But there's also a number of clinical trials, one just crossed my desk yesterday, or studies on humans that have recovered from infection showing the types of responses that are correlated. And yesterday's papers, how important cellular immune response is to the nuclear capsid are for these patients. So it's really lining up well. We've been talking about this for about 18 months to two years now, and finally getting a lot of traction and it's -- we kind of joke a little bit.
It's nice to see these animal studies validating what we already knew and are seeing in clinical trials. So I think there's a lot of opportunities here. How to be used in the end, some regulatory questions and things to address, as well. Because there's always going to be that standard of care product that's out there, and with each individual patient population, there might be a unique approach to the how you would administer the vaccine in combinations, as David said, heterologous prime-boost; or as individual products; yearly boosters; every five-year boosters, all those will probably have to be fine-tuned.
Leah Lough: I'm sorry if you're going to repeat yourself with my next question, but I just wanted to know if I may be missed if there were any changes to any of that current catalyst?
David Dodd: I was going to add, Leah, on that first issue, about the immunocompromised. Based on the discussions we're having with various advisors, consultants, we believe that there may very well be expedited pathways for focusing on certain immunocompromised populations, relative to the continued development of our COVID-19 vaccine, the CMO4S1. And we'll be further refining those through discussions, not just amongst ourselves and consultants, but also with the FDA, as we go forward into 2023, and as we start sharing the initial data results that we're getting from the existing trials. So we'll keep everyone updated on that.
But we do believe that by focusing specifically on populations with the immunocompromised, there may be expedited pathways and in much stronger points of differentiation for us than, obviously, going toe-to-toe with the very large players who could -- dealing with relatively healthy people. They're going to continue to have their challenges of addressing variants of concern, but we do believe that there is a strong point of distinction, differentiation that will be -- bode very well for GeoVax.
Question 3- Robert LeBoyer, Noble Capital: I was going to ask about the current environment for COVID vaccines and requirements for approval as the virus is evolving and variants are coming out, specifically in terms of what the regulatory path might be, and in terms of size of trials, next trials, or any light that you can shed on the next steps, after the phase 2 data comes out for either trial.
David Dodd: I think our anticipation is that that will be clarified as we engage in discussions, having data from these trials as they're coming out, with the FDA. Now, what we will be also addressing is the advice we're receiving, and we believe in it also rather strongly, is that there are certain populations for which we may have a very compelling argument or proposal to make to the regulatory agencies about targeting specific populations who clearly are not being well served, or served at all, by the current vaccines, even if they modify them to chase after a new, emerging variant.
And we plan to focus on that very strongly as we gather the data that we'll begin to see in the not too distant future and will engage in those discussions. And then, as we learn more, we'll keep you updated, but that's sort of the basis of the pathway that we're looking at.

David Dodd: This concludes our question-and-answer session. So what I'd like to do now is thank everybody for participating in this update call, sharing our achievements, our progress, and our outlook. We're quite excited about what is being achieved and what we're advancing here at GeoVax, and in a relatively short time. It was approximately just a little over two years ago that we did the recapitalization of the list from NASDAQ. And your interest is greatly valued and appreciated. So thank you.
Going forward, our focus is on reporting updates and progress for Gedeptin, for CMO4S1, and our other development programs, as well as expansion of our capabilities and resources. Our goal and focus is to build shareholder and stakeholder value.
I want to also acknowledge and thank our Board of Directors, our GeoVax staff, and the many other parties that continue to support, assist, and advise us towards achieving success. For all of us, it is indeed a great pleasure serving our shareholders and being a part of this team and on the pathway that we're pursuing. So have a safe, enjoyable day and again, thank you for your interest.
GeoVax Labs, Inc. is a clinical-stage biotechnology company developing novel therapies and vaccines for cancers and many of the world’s most threatening infectious diseases. The company’s lead program in oncology is a novel oncolytic solid tumor gene-directed therapy, Gedeptin®, presently in a multicenter Phase 1/2 clinical trial for advanced head and neck cancers. GeoVax’s lead infectious disease candidate is GEO-CM04S1, a next-generation COVID-19 vaccine targeting high-risk immunocompromised patient populations. Currently in two Phase 2 clinical trials, GEO-CM04S1 is being evaluated as a single-dose COVID-19 vaccine for immunocompromised patients such as those suffering from hematologic cancers and other patient populations for whom the current authorized COVID-19 vaccines are insufficient. In addition, GEO-CM04S1 is in a Phase 2 clinical trial evaluating the vaccine as a more robust, durable COVID-19 booster among healthy patients who previously received the mRNA vaccines. GeoVax has a leadership team who has driven significant value creation across multiple life science companies over the past several decades. For more information, visit our website: www.geovax.com.
This release contains forward-looking statements regarding GeoVax’s business plans. The words “believe,” “look forward to,” “may,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,” “could,” “target,” “potential,” “is likely,” “will,” “expect” and similar expressions, as they relate to us, are intended to identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. Actual results may differ materially from those included in these statements due to a variety of factors, including whether: GeoVax is able to obtain acceptable results from ongoing or future clinical trials of its investigational products, GeoVax’s immuno-oncology products and preventative vaccines can provoke the desired responses, and those products or vaccines can be used effectively, GeoVax’s viral vector technology adequately amplifies immune responses to cancer antigens, GeoVax can develop and manufacture its immuno-oncology products and preventative vaccines with the desired characteristics in a timely manner, GeoVax’s immuno-oncology products and preventative vaccines will be safe for human use, GeoVax’s vaccines will effectively prevent targeted infections in humans, GeoVax’s immuno-oncology products and preventative vaccines will receive regulatory approvals necessary to be licensed and marketed, GeoVax raises required capital to complete development, there is development of competitive products that may be more effective or easier to use than GeoVax’s products, GeoVax will be able to enter into favorable manufacturing and distribution agreements, and other factors, over which GeoVax has no control.