Moderator: Certain statements in this presentation may constitute "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act. These statements are based on management's current expectations and are subject to uncertainty and changes in circumstances. Actual results may differ materially from those included in these statements due to a variety of factors, including whether: GeoVax can develop and manufacture its vaccines with the desired characteristics in a timely manner, GeoVax's vaccines will be safe for human use, GeoVax's vaccines will effectively prevent targeted infections in humans, GeoVax’s vaccines will receive regulatory approvals necessary to be licensed and marketed, GeoVax raises required capital to complete vaccine development, there is development of competitive products that may be more effective or easier to use than GeoVax's products, GeoVax will be able to enter into favorable manufacturing and distribution agreements, and other factors, over which GeoVax has no control. GeoVax assumes no obligation to update these forward-looking statements and does not intend to do so. More information about these factors is contained in GeoVax's filings with the Securities and Exchange Commission including those set forth at "Risk Factors" in GeoVax's Form 10-K.
It is now my pleasure to introduce the Chairman and CEO of GeoVax, David Dodd.
DAVID DODD: Good afternoon and thank you for participating in the 2021 Fourth Quarter/Year-End update call.
We are pleased to have this opportunity to review our successful acceleration into Phase 2 clinical development within Covid-19 and immuno-oncology, while we continue to secure critical resources in support of GeoVax growth and development, while advancing our IND-enabling programs.

Over the past year, we utilized our balance sheet to catapult into Phase 2 clinical development through the strategic in-licensing of both GEO-CM04S1 and Gedeptin®.
CM04S1, which we in-licensed exclusive global rights from City of Hope National Medical Center, is a next generation Covid-19 vaccine, targeting both antibody and cellular immunity with the goal of providing more robust and durable protection than the current authorized vaccines.
Gedeptin is a cancer immunotherapy, which we in-licensed exclusive global rights from PNP Therapeutics and the University of Alabama (Birmingham), currently being evaluated among patients suffering from advanced Head & Neck cancers and has received Orphan Drug designation from the FDA.
In addition, we’re advancing encouraging internal programs on the path to IND-filing.
In January, we issued a 2021 milestone report, addressing the goals we established and communicated early last year. Entering 2021, we built a strong balance sheet supporting both strategic transactions and an organization with the expertise to accelerate our growth and development. We successfully executed our plans, propelling our status into Phase 2 clinical development within both Covid-19 and immune-oncology. In addition, we advanced our internal programs within Covid-19 and immuno-oncology, as well as reported encouraging results in support of our hemorrhagic fever virus vaccine program.
This included a scientific presentation at the 2021 World Vaccine & Immunotherapy Congress in early December, validating our Covid-19 vaccine approach of a multi-antigenic vaccine eliciting strong antibody and cellular immune responses potentially providing more robust and durable protection beyond the current authorized vaccines which are primarily designed to induce neutralizing antibodies.
In fact, in a well-validated lethal challenge transgenic mouse model, our CM02 candidate, which is the first step towards a universal coronavirus vaccine, provided complete protection following a single dose, even in the absence of measurable neutralizing antibodies. To our knowledge, these results are unprecedented. I’ll note that both our Phase 2 Covid-19 vaccines, CM04S1, and our CM02 both are multi-antigenic Covid-19 vaccines, designed to strongly elicit both antibody and cellular immune responses. At the same scientific congress, we also reported further, encouraging results in support of our Marbug and Sudan vaccines. During this year, we anticipate reporting milestones and progress related to both our clinical and IND-enabling programs. So, let’s move into further discussion related to our exciting programs and activities underway at GeoVax.

As we entered this year, we further strengthened our balance sheet, while focusing on the acceleration of our CM04S1 and Gedeptin Phase 2 programs and completing the IND-enabling activities in support of our CM02 vaccine for Covid-19, our MUC1 vaccine in immuno-oncology and our Hemorrhagic Fever Virus vaccines, the latter of which are supported via non-dilutive funding and activities. In addition, we have further enhanced our resources and expertise in support of successfully executing upon our 2022 and beyond plans for growth and development of GeoVax.
Our priority focus in 2022 is to accelerate the recruitment and enrollment of our three Phase 2 programs. This includes our multi-site clinical trial in support of Gedeptin and our two clinical trials in support of CM04S1.

Since our recent Gedeptin announcement, we have confirmed two additional clinical sites and the assignment of CATO SMS as our CRO partner responsible for leading the expansion and acceleration of the Gedeptin clinical program. Our goal is to complete patient enrollment towards the end of 2022 or early 2023, followed by completion of patient evaluations by the end of 2023. Should the results be supportive, a BLA filing will likely shortly thereafter. In parallel with the ongoing clinical program, we are also engaged with a CDMO to prepare for commercial manufacture.
We are confident that the Gedeptin Phase 2 program will be successfully managed by CATO SMS and our Clinical Operations team, with possible expansion of further additional clinical sites soon.
We are highly excited about the outlook and promise of Gedeptin within advanced Head & Neck cancer, where it has received Orphan drug designation from the FDA and has previously provided encouraging potential for such patients. In addition, there are promising opportunities relative to expanded use of Gedeptin in other indications, as well as the GDPT technology in conjunction with other therapies and, potential synergy with our MVA-VLP tumor associated antigen approach. We are looking forward to providing milestone updates throughout this year about the progress of our Gedeptin program.
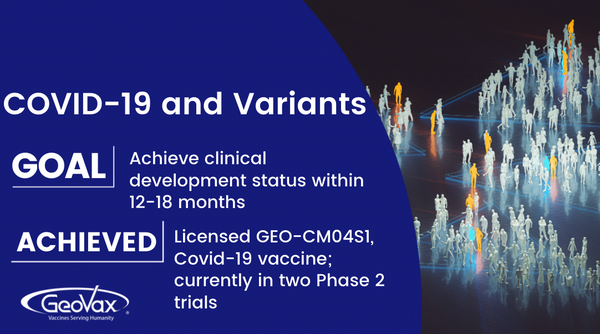
In November, we announced the exclusive world-wide license of a Phase 2 COVID-19 vaccine from City of Hope National Medical Center. This exciting vaccine is now referred to as GEO-CM04S1, which we typically refer to as “CM04S1”. CM04S1 utilizes a synthetic modified vaccinia Ankara (MVA) technology, similar to our other vaccine programs under development at GeoVax.
This transaction propelled us into a critical stage of clinical development. CM04S1 works by inducing immunity to SARS-CoV-2 by stimulating the immune system to produce antibodies against SARS-CoV-2 that can block the virus from entering healthy cells, while the immune system can also grow new disease-fighting T cells that can recognize and destroy infected cells.
The vaccine includes both SARS-CoV-2 spike and nucleocapsid proteins. By inserting these proteins, the MVA delivery vehicle is able to drive the expression of both proteins within the body of the vaccine recipient, inducing immune responses. The role of the S-protein is to elicit a neutralizing antibody response against the initial infection, while the N-protein elicits a T-Cell response to directly attack virus infected cells, reduce viral replication and reduce severity and clearance. Thus, the vaccine is designed to induce both neutralizing antibodies and T-cell responses specific for the S-protein and the N-protein.
This vaccine design was implemented specifically to induce an expanded immune response to better combat and clear infections regardless of the circulating SARS-CoV-2 variants. Our goal is to provide a vaccine that “gets ahead of the variants” versus having to “chase the variants” which, if successful, will reduce reliance on the repeated administration of booster doses of existing vaccines.
We believe that such a “multi-punch” approach has the potential for providing a more robust and durable immune response and protection than the current authorized vaccines. We also believe that various high-risk populations, such as immune-compromised individuals, will benefit from such a two-prong approach.

CM04S1 is currently being evaluated in two Phase 2 clinical trials. One trial is the first comparative study of an investigational COVID-19 vaccine with the current Food and Drug Administration (FDA)-approved Pfizer vaccine in people that have received or are undergoing specific blood cancer therapies associated with transplantation or CAR-T therapy that suppress or severely reduce pre-existing immunity to COVID-19 vaccines. Multiple clinical trials have demonstrated that such patients fail to respond optimally to the current generation vaccines and we believe the CM04S1 will prove to be more potent because it is multi-antigenic and delivered using the MVA vector. We believe this will differentiate CM04S1 from the other vaccines by providing both a strong antibody response and a sustained T cell response in these patients who are still at high risk, due to their immunocompromised status, of severe COVID-19.

The other trial currently underway is evaluating CM04S1 as a booster for healthy patients who have previously received either the Pfizer or Moderna mRNA vaccines. We believe that providing a heterologous booster, rather than a 3rd or 4th shot of the same vaccine, may provide more robust and durable immune response and protection. Heterologous prime-boost immunizations are well studied in other fields, such as HIV, and are being evaluated in multiple countries using different COVID vaccines. We are working with CATO SMS and Pharm-Olam, following their recent merger, to oversee the acceleration and management of these two exciting clinical programs, working in conjunction with our Clinical Operations team.
Finally, the ongoing GeoVax effort to develop a manufacturing process based on a continuously growing avian cell line to increase production consistency and capacity will mesh with the clinical development activities and full development schedule associated with the CM04S1 and CM02 vaccines. The potential benefits of this transaction to the GeoVax program are highly significant and timely.
Now, I’d like to turn the presentation over to Mark Reynolds, GeoVax Chief Financial Officer for a review of our recent results and financial status.
MARK REYNOLDS:
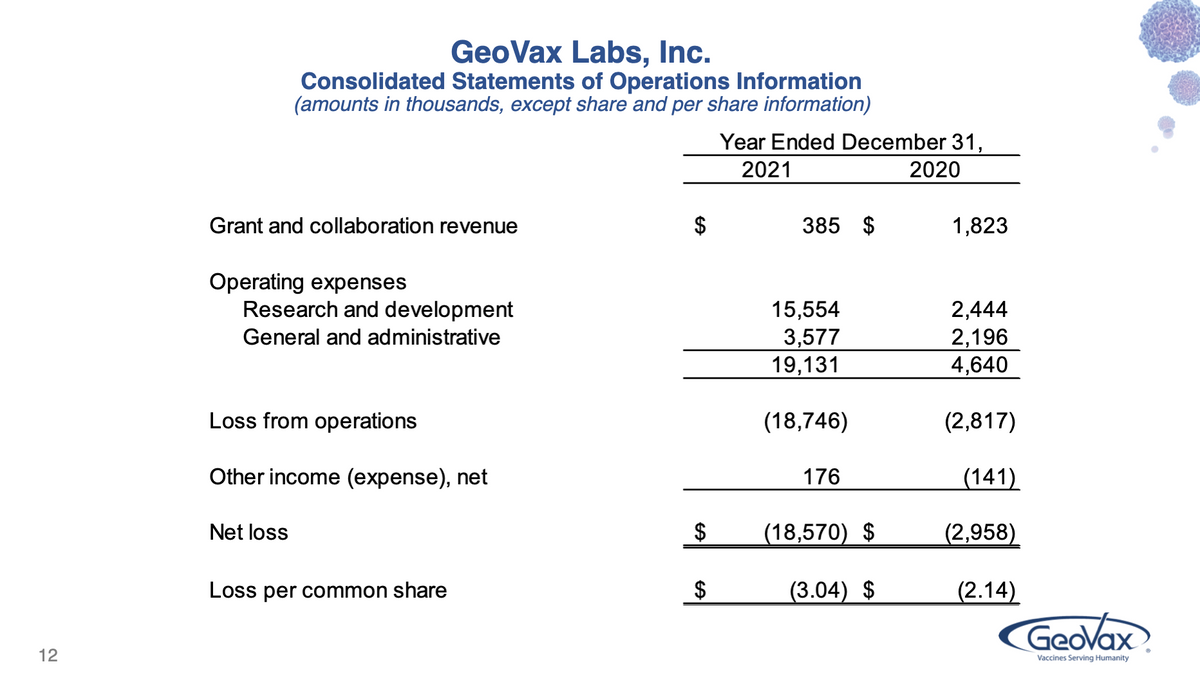
Starting with our Income Statement.... Grant and collaboration revenues were $385,000 during 2021 vs $1.8 million in 2020. The 2021 period revenues relate entirely to our grant from the NIH supporting our COVID-19 vaccine, while the 2020 amount includes revenues from our grant from the U.S. Army supporting our Lassa Fever vaccine program, which began to wind down during the year.
Research and development expenses were $15.6 million in 2021 vs $2.4 million in 2020, with the increase primarily associated with our license fees (paid and accrued) and clinical trial expenses related to our license agreement with City of Hope for our COVID-19 vaccine program as well as our license of Gedeptin. Also contributing to the increase were expenses related to our universal coronavirus vaccine program, manufacturing process development, and a generally higher level of activity.
General and administrative expenses were $3.6 million in 2021 vs $2.2 million in 2020. A large portion of the increase here relates to the annual Delaware franchise tax, which is based on our capitalization and was minimal in 2020. Other increases in insurance premiums, patent costs, legal fees, consulting fees, and personnel costs are generally associated with preparing the organization for a higher activity level following our capital raises.
Other income/expense for 2021 includes a $172,000 gain on extinguishment of debt, associated with the forgiveness of the PPP loan we received in early 2020. The comparable figure for 2020 includes interest expense of $144,000 related to interest and debt discount amortization from convertible debentures that were retired in 2020.
So, overall net loss for 2021 was $18.6 million (or $3.04 per share) vs $3.0 million in 2020 (or $2.14 per share), again with the increase primarily associated with our licensing and R&D activities.
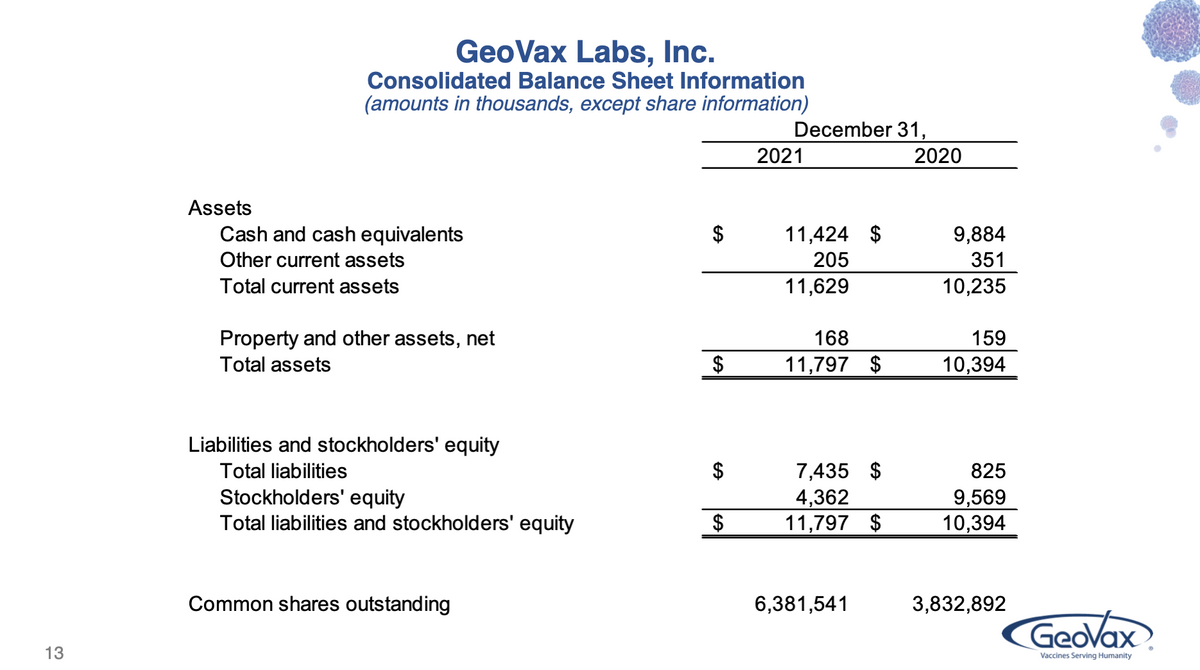
Turning now to our Balance Sheet, cash balances at Dec 31 were $11.4 million, as compared to $9.9 million at Dec 31, 2020. The change in our cash balances is reflective of $11.2 million used in operating activities, offset by proceeds from a stock offering in early 2021, with net proceeds to us of $9.4 million. We also received $3.4 million from the exercise of our publicly-traded warrants.
Subsequent to year-end, in January we raised an additional $9.2 million through a private placement priced at $3.26 per share. So as of today, GeoVax has cash balances of approximately $17 million.
Now that we have 3 ongoing Phase 2 clinical programs in COVID-19 and immuno-oncology, our cash needs have obviously increased, not only for license fees and direct costs associated with the clinical programs, but also for the facilities, personnel, and other costs to support those programs. While we don’t provide specific forward-looking estimates of the costs to complete our research programs, what we can say at this time is that our existing cash reserves are sufficient to fund our operations and priority programs into the second quarter of 2023. We believe the advancement of these programs will create an attractive investment opportunity for new fund-raising activities.
Closing remarks:
DAVID DODD:
Thank you everyone for participating in this conference call, sharing in our milestone achievements resulting in clinical stage development within both immuno-oncology and SARS-CoV-2. But, for us, we’ve just started as we accelerate our pace of development and progress.
We look forward to updating you throughout 2022 as we report milestones for Gedeptin, CM04S1, CM02 and, our other development programs, as well as expansion of our capabilities and resources. Our mission is to provide products that prevent or treat some of the world's most challenging infectious diseases and cancers, by leveraging technology and collaborations that allow us to successfully provide life-enhancing products in a safe, scientific-validated manner. Our business belief is simple: Science is Global and should always serve Humanity first. To that end, our pursuit is to deliver safe, affordable products to improve lives worldwide, delivering increased value to our shareholders and providing motivating career development opportunities to members of our team. We firmly believe that GeoVax can provide differentiated, advanced vaccines and immunotherapies, competing with and collaborating with other companies worldwide. We intend to successfully execute in this regard.
Finally, I want to acknowledge and thank the GeoVax Board of Directors, our GeoVax staff and the many other parties that continue to support, assist and advise us towards achieving success.
For all of us, it is a great pleasure serving our shareholders and being a part of this team. Have a safe and enjoyable day.
GeoVax Labs, Inc. is a clinical-stage biotechnology company developing human vaccines and immunotherapies against infectious diseases and cancer using novel proprietary platforms. GeoVax’s product pipeline includes two ongoing Phase 2 clinical trials of GEO-CM04S1 (formerly COH04S1) for COVID-19 as a universal booster vaccine to mRNA vaccines authorized by the U.S. Food and Drug Administration (FDA) and as a primary vaccine for use in immunocompromised patients. In addition to GEO-CM04S1 for COVID-19, GeoVax is developing GEO-CM02 as a pan-coronavirus vaccine. The Company is also conducting a Phase 1/2 clinical trial of Gedeptin® for treatment of head and neck cancer. Gedeptin® has been granted orphan drug status by the FDA. Additional research and development programs include preventive vaccines against Zika Virus, hemorrhagic fever viruses (Ebola, Sudan, Marburg, and Lassa) and malaria, as well as immunotherapies for multiple solid tumors. The Company’s portfolio of wholly owned, co-owned, and in-licensed intellectual property stands at over 70 granted or pending patent applications spread over 20 patent families.
This release contains forward-looking statements regarding GeoVax’s business plans. The words “believe,” “look forward to,” “may,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,” “could,” “target,” “potential,” “is likely,” “will,” “expect” and similar expressions, as they relate to us, are intended to identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. Actual results may differ materially from those included in these statements due to a variety of factors, including whether: GeoVax is able to obtain acceptable results from ongoing or future clinical trials of its investigational products, GeoVax’s immuno-oncology products and preventive vaccines can provoke the desired responses, and those products or vaccines can be used effectively, GeoVax’s viral vector technology adequately amplifies immune responses to cancer antigens, GeoVax can develop and manufacture its immuno-oncology products and preventive vaccines with the desired characteristics in a timely manner, GeoVax’s immuno-oncology products and preventive vaccines will be safe for human use, GeoVax’s vaccines will effectively prevent targeted infections in humans, GeoVax’s immuno-oncology products and preventive vaccines will receive regulatory approvals necessary to be licensed and marketed, GeoVax raises required capital to complete development, there is development of competitive products that may be more effective or easier to use than GeoVax’s products, GeoVax will be able to enter into favorable manufacturing and distribution agreements, and other factors, over which GeoVax has no control.